Temperature Changes in an Ideal Gas
Temperature Changes in an Ideal Gas
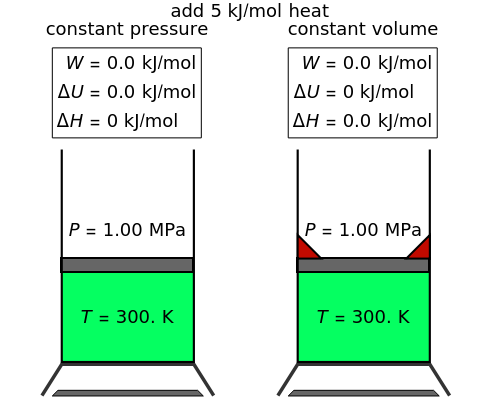
In this Demonstration, heating or cooling of an ideal gas is compared at constant pressure and constant volume. You can vary the amount of heat added (for "heat gas") or removed (for "cool gas"). Clicking the play button to the right of "heat gas" or "cool gas" calculates the final temperature , the work done on the gas, the change in internal energy , and the change in enthalpy for each system. The final pressure is also calculated for the constant-volume system.
T
W
ΔU
ΔH
P