Separation of a Partially Miscible Mixture Shown by Graphical Method
Separation of a Partially Miscible Mixture Shown by Graphical Method
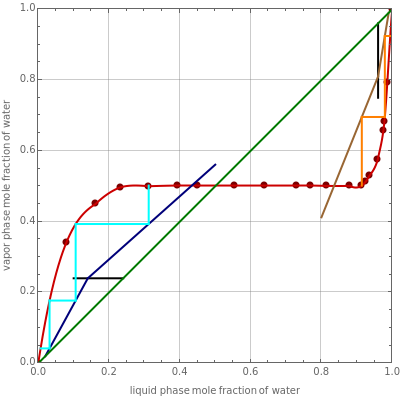
Consider a binary mixture of water and nitromethane at 1 atm. This mixture exhibits the vapor-liquid equilibrium behavior of partially miscible mixtures (equilibrium data is available in [1]). Thus, in order to separate water from nitromethane, one has to use a two-column system with an intermediate decanter. This Demonstration shows the required operational setup, which will produce two product streams: a pure water stream and a water-free stream.
()
CH
3
NO
2
Assume that the feed to the first column is a saturated vapor containing water flowing at and that the feed to the second column is a saturated liquid containing water flowing at . The bottom compositions are set to water and water for columns 1 and 2, respectively.
24mole%
1000kg-mole/day
96mole%
500kg-mole/day
2mole%
99mole%
External mass balances give the flow rates of the bottom products of both columns, =788.66 and =711.33 .
B
1
B
2
kg-mole/day
The Demonstration uses the McCabe and Thiele graphical method to compute the number of stages for both columns. You can set the two reboil ratios.