Second-Order Reaction with Diffusion in a Liquid Film
Second-Order Reaction with Diffusion in a Liquid Film
Gas absorption is often enhanced by a chemical reaction. For instance, acid gases ( and S) are usually eliminated from natural gas by absorption using ethanolamine (OH) as a basic solvent.
CO
2
H
2
H
2
NC
2
H
4
Consider the absorption of species with a solvent containing a species such as a second-order irreversible chemical reaction, , that takes place in a liquid film. Only species is present in the gas phase since has a very low vapor pressure (i.e., is a high boiling component). Species is not present in the bulk liquid since all of reacts with component in the liquid film.
A
D
B
A+BC
A
B
B
A
A
B
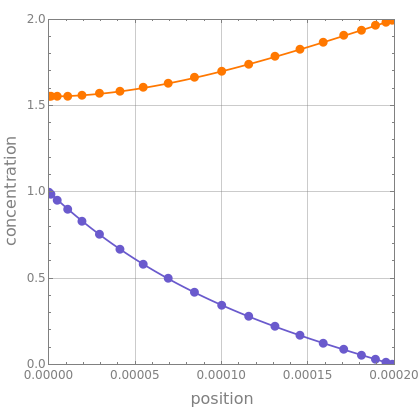
he steady state material balances within the film are given by =k and =k, where the binary diffusion approximation for and in has been used. These equations simply state that the rates of diffusion of species and are equal to the rate of the chemical reaction. The concentration of in the bulk liquid is arbitrarily set to 2 while the concentration of at the gas-liquid interface is set to 1.
D
AD
2
d
c
A
d
2
x
c
A
c
B
D
BD
2
d
c
B
d
2
x
c
A
c
B
A
B
D
A
B
B
A
This Demonstration displays the liquid film concentrations of species and (blue and orange curves, respectively) as a function of position. You can change the values of the diffusivities, and , the reaction rate constant, , as well as the number of Chebyshev collocation points, . Excellent agreement is obtained between the numerical solutions given by Chebyshev orthogonal collocation (blue and orange dots) and by NDSolve (blue and orange curves).
A
B
D
AD
D
BD
k
N+1