Oxidation of Ammonia by Air
Oxidation of Ammonia by Air
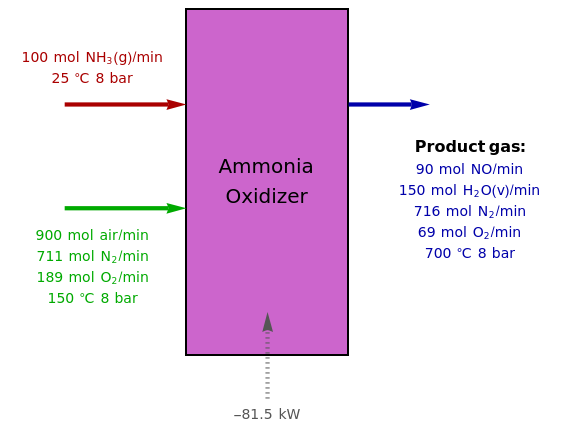
Ammonia is oxidized by air to form nitric oxide, as the first step in the production of nitric acid. The two principal reactions that occur are (reaction R1) and (reaction R2). The inlet and outlet conditions are given in the reactor's flowchart. You can set the temperature of the outlet stream. The extent of reaction R1 is =22.5mol/min, while the extent of reaction R2 is =5.0mol/min.
4(g)+5(g)→4NO(g)+6O(v)
NH
3
O
2
H
2
2(g)+(g)→(g)+3O(v)
NH
3
3
2
O
2
N
2
H
2
ξ
1
ξ
2
The standard enthalpies of reactions R1 and R2 are and , respectively. All of the heat requirements for the formations are obtained from [1, Appendix B1].
Δ=-904.74kJ/mol
0
H
r,1
Δ=-633.11kJ/mol
0
H
r,2
This Demonstration computes the heat added to the reactor in kW. For this purpose, we make use of the energy balance equation for reactive systems: =Δ=Δ+Δ+- (i.e. the heat of reaction method). The specific enthalpies of all components are computed using the temperature-dependent expressions of the constant-pressure heat capacities given in [1, Appendix B2]. In addition, we chose as reference states the molecular species , (g), (g), (g), and O(v) at 25 °C and 1 atm.
Q
Q
H
ξ
1
0
H
r,1
ξ
2
0
H
r,2
∑
i
out
n
i
H
i
∑
i
in
n
i
H
i
Air(g)
N
2
O
2
NH
3
NO(g)
H
2