Measuring the Specific Heat of a Substance with a Calorimeter
Measuring the Specific Heat of a Substance with a Calorimeter
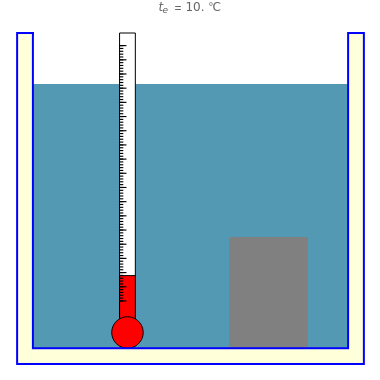
A substance of mass with a temperature is submerged into a calorimeter with 600 g of water initially at 4 °C. After some time, the system reaches an equilibrium temperature that determines the specific heat of the substance. The heat lost by the substance is gained by the water. For accurate results, the system should be thermally insulated.
m
t
0
t
e