Mayo-Lewis Model for Copolymer Composition
Mayo-Lewis Model for Copolymer Composition
Copolymer composition is a significant parameter in determining the properties of the copolymer, and ultimately, its practical applications. To predict copolymer composition during the polymerization process, one has to determine the concentrations of the monomers in the reactor and their reactivities.
Mayo and Lewis [1] developed a simple equation to measure the instantaneous copolymer composition as a function of both the feed composition and the monomers' reactivity ratios. This model is based on the following four possible propagation reactions:
*
M
A
M
A
k
AA
→
*
M
A
*
M
A
M
B
k
AB
→
*
M
B
*
M
B
M
B
k
BB
→
*
M
B
*
M
B
M
A
k
BA
→
*
M
A
where and are the monomers and , respectively, and are growing chains terminating with and units, respectively, and , , , and are propagation rate constants.
M
A
M
B
A
B
*
M
A
*
M
B
A
B
k
AA
k
BB
k
AB
k
BA
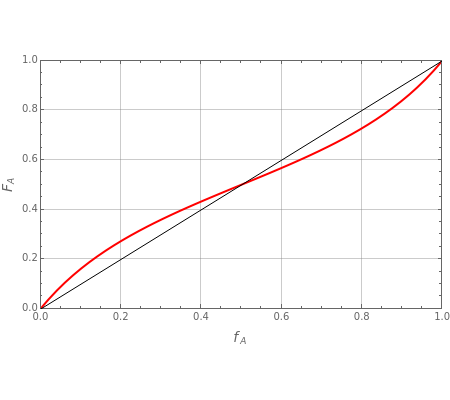
The final expression for the Mayo–Lewis equation is:
F
A
r
A
2
f
A
f
A
f
B
r
A
2
f
A
f
A
f
B
r
B
2
f
B
where and are the mole fractions of the monomers in the feed , is the mole fraction of formed in the copolymer, and and are the reactivity ratios given by: = and =.
f
A
f
B
(=1-)
f
A
f
B
F
A
A
r
A
r
B
r
A
k
AA
k
AB
r
B
k
BB
k
BA
The copolymer structures can be predicted by knowing the absolute values of the reactivity ratios. For example:
1. If is close to 1, then the resultant copolymer has an ideal random structure. The copolymerization of methyl methacrylate and methacrylamide is a clear example of such a system (=3.2 and =0.3). Most of the copolymers are in this category and thus have the tendency to be random. Styrene, with a reactivity ratio of , and butadiene, with a reactivity ratio of , are other examples of random copolymers.
r
A
r
B
r
MMA
r
MAM
0.8
1.4
2. If at least one of the reactivity ratios is equal to zero, then the resulting copolymer has an alternating structure. For example, for the copolymerization involving a two-component system composed of styrene and maleic anhydride, both reactivity ratios are equal to zero.
Similarly to a bicomponent liquid mixture, azeotropic compositions exist in some copolymerization processes. This azeotropic composition leads to a composition drift in the final product. If both reactivity ratios are equal to one, then all the values of in the Mayo–Lewis equation have azeotropic composition. For more information about the subject, the reader can consult the excellent textbook by Rudin [2].
f
A