Flash Distillation Cascade for an Acetone-Chloroform Mixture
Flash Distillation Cascade for an Acetone-Chloroform Mixture
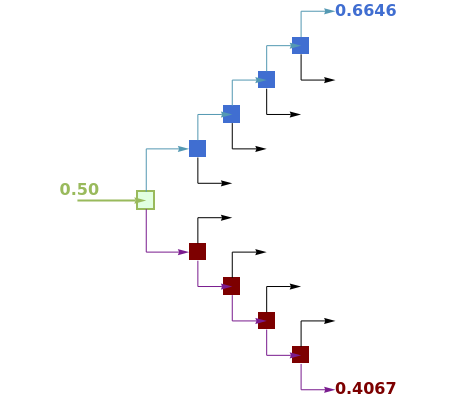
A binary mixture of acetone and chloroform is fed to a flash distillation cascade. This mixture contains a user-set value of mole % acetone. This binary mixture presents a negative azeotrope with 34.07 mole % of acetone at a boiling point of 64.49 °C. Vapor-liquid equilibrium data is generated using a modified Raoult's law with activity coefficients predicted by the van Laar model.
This Demonstration presents the flash distillation cascade that gives a liquid stream (with a composition approaching the azeotropic composition when the number of flash operations is large enough) and an acetone-rich or chloroform-rich vapor stream (depending on the user-set feed composition). The compositions of these two streams are shown in red and blue, respectively.
Temperature is decreased in the rectifying cascade in order to produce the desired top vapor stream (an acetone-lean or a chloroform-lean product) and increased in the stripping cascade in order to obtain a liquid stream with mole fractions approaching the azeotropic composition. The gradual temperature changes are adjusted so that the vapor fraction in the feed of any flash vessel is always 50%.
The simulations show in particular that pure acetone and pure chloroform cannot be obtained simultaneously even if you use a very large number of flash distillation vessels.
The VLE diagram selection shows the temperature changes as red and blue arrows as well as the location of the vapor streams in the rectifying cascade (shown as cyan dots). Finally, the liquid streams in the stripping cascade are shown using magenta dots.