Chemical Reactions Described by the Lorenz Equations
Chemical Reactions Described by the Lorenz Equations
This Demonstration analyzes the behavior of a chemical reaction scheme that is described by the Lorenz equations:
dX
dt
dY
dt
dZ
dt
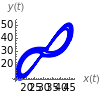
Parameters that lead to interesting behavior are ; the strange attractor that evolves from these equations spans both positive and negative values of and . If we interpret these symbols as representing concentrations of chemical species, they cannot be negative; a shift of the and axes can give new variables that are always positive [1]. Thus if we choose , , with , the equations describing the system become
(a,b,c)=(10,28,8/3)
X
Y
X
Y
x=X+δ
y=Y+δ
z=Z
δ=30
dx
dt
dy
dt
dz
dt
2
δ
x
y
z
dx
dt
dy
dt
dz
dt
8
3
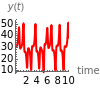
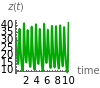
The reactions required to give the Lorenz equations are shown in section (1.7) of [1]. You can vary the time and the initial values of the species to see the evolution of the system. There is an unstable steady state at and an unstable fixed point at .
(x,y,z)=(38.485,38.485,27)
(x,y,z)=
72
+30,72
+30,27