Bose-Einstein, Fermi-Dirac, and Maxwell-Boltzmann Statistics
Bose-Einstein, Fermi-Dirac, and Maxwell-Boltzmann Statistics
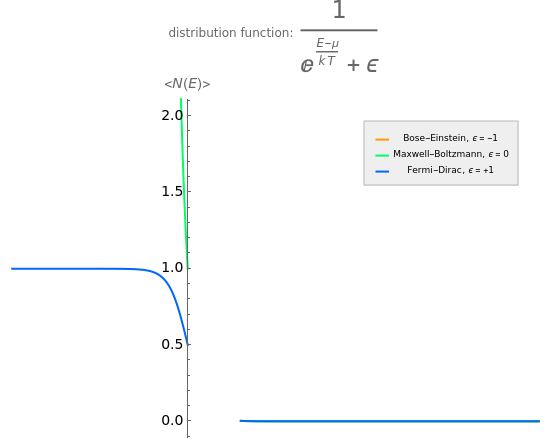
This is a plot of the population density of the Bose–Einstein, Fermi–Dirac, and Maxwell–Boltzmann thermodynamic statistics. The coordinate is , where is the chemical potential.
<N(E)>
x
E-μ
μ
The thermodynamic chemical potential is the change of a characteristic state function per change in the number of particles. More precisely it is the thermodynamic conjugate of the particle number. It can be interpreted, for example, as the ability of the system to perform phase transitions or chemical reactions, or its tendency to diffuse. In the case of massless particles, such as a gas of photons, because the number of particles in the ensemble is not conserved.
μ
μ=0
The Fermi–Dirac statistics describe the population density of a gas of fermions. As it becomes a step function; the fermions in the gas populate states with an energy ≤μ, while states with >μ are not occupied. The reason is that fermions obey Pauli's exclusion principle, which states that two particles cannot populate the same state at the same time.
T0
E
n
E
n
The Bose–Einstein statistics describes a gas of bosons. Since they do not obey Pauli's exclusion principle, the same state can be populated by more than one particle; the distribution function diverges for .
Eμ
Maxwell–Boltzmann statistics apply where quantum-mechanical effects do not play a role and the particles of the gas can be considered "distinguishable". Both Fermi–Dirac and Bose–Einstein statistics become Maxwell–Boltzmann statistics at high temperatures and low chemical potentials where .
μ<0