Case 1: pure chemistry
Case 1: pure chemistry
All that matters is molecular species. Generate a multiway graph of possible reactions. Then use path counting etc. to find “concentrations”
Case 2: subchemistry
Case 2: subchemistry
Account for individual molecules. E.g. there might be a trillion of some type of molecules. Each individual molecule is tracked in subchemistry. [ Molecular dynamics ]
E.g. everything is on a grid. Molecules move
As soon as we have many molecular types, we can make a causal graph
Note: in general the interactions could have many outcomes, i.e. a multiway graph. [This is what we need for stochastic / quantum stuff]
Modeling chemical species / reactions
Modeling chemical species / reactions
Strings [ or trees or graphs ]
Strings [ or trees or graphs ]
Typically we have local substitutions on the strings
We can have multiway-ness either because of different places to hit the strings, or multiple outcomes from a given rule
Strings + space
Strings + space
Strings + space + background configuration
Strings + space + background configuration
Spatialization
Spatialization
[E.g. Chemistry-01 ]
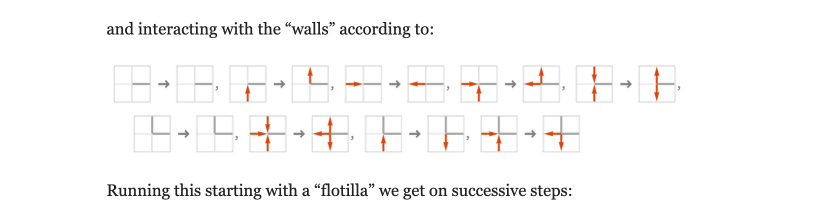
Simplest case: block cellular automaton (1D, 2D, ...)
Out[]=
,
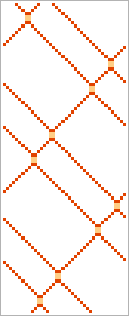
This sequence is like going from gas to liquid
Out[]=
,
,
,
Rules don’t have to be reversible in chemistry...
Look at 2D case as well....
[[ Given strings we effectively have an unbounded number of species ... ]]