Blackbody Radiation
Blackbody Radiation
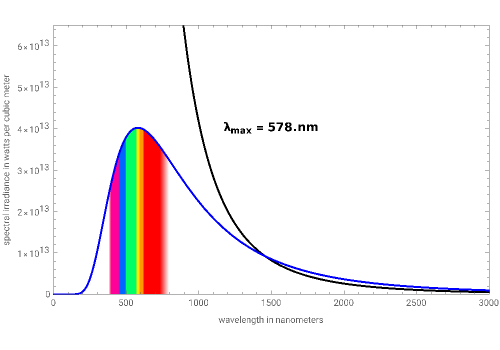
Blackbody radiation is the maximum amount of energy an object can emit. This Demonstration shows how Max Planck was able to close the gap between the explanation of classical physics and the observed experimental data. His formula was derived by assuming that oscillating molecules emit energy as quantized discrete values. His findings and Einstein's later discovery of photons led to the basis for quantum mechanics.
Rayleigh–Jeans law (classical model): =(2ckT)/
I
λ
4
λ
Planck's law: =(2hπ)((-1+))
I
λ
2
c
(ch)/(kTλ)
E
5
λ
where
= spectral irradiance: emitted radiation intensity/wavelength )
= speed of light
= Planck's constant
= Boltzmann constant
= wavelength (m)
= temperature (K)
I
λ
(W/
3
m
c
h
k
λ
T
Details
Details
From the snapshots, you can see that as the temperature increases, the maximum wavelength decreases since the blackbody emits higher frequency/energy photons. The distribution also becomes less spread out at higher temperatures as the radiated energy becomes more focused around the maximum intensity wavelength.
Reference: S. O. Kasap, Principles of Electronic Materials and Devices, 3rd ed., Boston: McGraw-Hill, 2006.
External Links
External Links
Permanent Citation
Permanent Citation
Zach Heuman
"Blackbody Radiation"
http://demonstrations.wolfram.com/BlackbodyRadiation/
Wolfram Demonstrations Project
Published: March 7, 2011