Flash Distillation of a Mixture of Four Hydrocarbons
Flash Distillation of a Mixture of Four Hydrocarbons
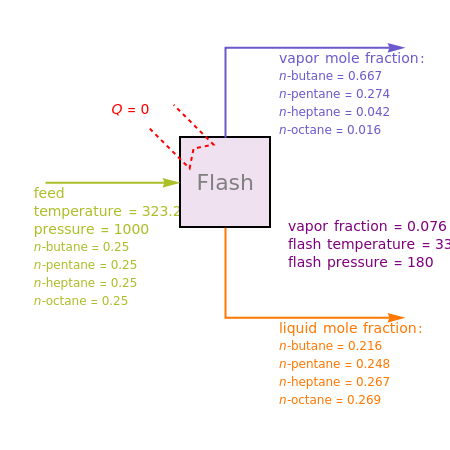
Consider a liquid feed composed of -butane, -pentane, -heptane, and -octane at 50 °C and 1000 kPa. The feed has a flow rate of 10 kmol/hr and contains mole% for each of the four hydrocarbons.
n
n
n
n
25
The Demonstration calculates the composition of the liquid and vapor leaving the flash vessel for user-selected values of the flash pressure, , and the added heat rate, , expressed in kJ/hr. The adiabatic flash distillation case is recovered if one sets . Values of the flash pressure (in kPa) and temperature (in Kelvin) are also displayed.
P
Q
Q=0
Details
Details
Fugacity coefficients as well as enthalpy correction functions are calculated using the Peng–Robinson equation of state[1, 2].
References
References
[1] E. J. Henley and J. D. Seader, Equilibrium-Stage Separation Operations in Chemical Engineering, New York: Wiley, 1981.
[2] D-Y. Peng and D. B. Robinson, "A New Two-Constant Equation of State," Industrial and Engineering Chemistry Fundamentals, 15(1), 1976 pp. 59–64. DOI: 10.1021/i160057a011
Permanent Citation
Permanent Citation
Housam Binous, Naim Faqir, Brian G. Higgins
"Flash Distillation of a Mixture of Four Hydrocarbons"
http://demonstrations.wolfram.com/FlashDistillationOfAMixtureOfFourHydrocarbons/
Wolfram Demonstrations Project
Published: November 27, 2012