Kinetics of Elimination Reactions
Kinetics of Elimination Reactions
This Demonstration shows the various types of elimination reactions involving alkyl halides.
There are three cases, depending on whether the hydrogen in the -position detaches earlier (E1cB), simultaneously (E2) or later than the halogen of the molecule (E1).
β
Select "planarity check" to highlight the planarity of the atoms, which is associated with hybridization .
2
sp
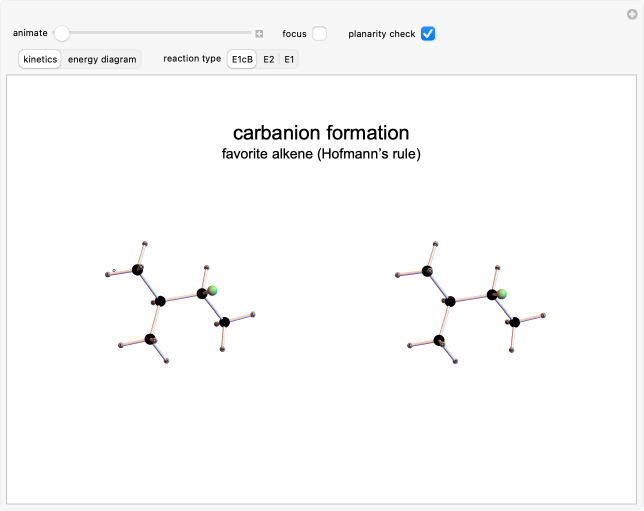
Select "focus" to highlight the inductive effect (+I) of the methyl groups. This effect destabilizes carbanions but stabilizes carbocations and molecules with double bonds. This stabilization is responsible for the application of Zaitsev's rule or Hofmann's rule[1, 2].
After enabling the elimination reaction, use "kinetics" and "animate" controls to show the progression of the reaction.
Select the elimination reaction "energy diagram" to study reaction coordinates related to the three possible elimination reactions: both E1cB and E1 reactions occur in two steps (intermediate reaction and two transition states), while the E2 reaction takes place in a single step (with a single transition state).
All three reactions are under kinetic control. The product ratio is determined by the rate at which the products are formed, so in the E1cB reaction, the product with the lowest activation energy is formed most rapidly[1–3]. The most energetically favored path is colored in blue, the least favored in red. Spheres moving along the reaction path represent the products, which are formed faster and in greater quantities due to the lower activation energy of the first step of the reaction.
Details
Details
The elements are represented with the following color code:
-H | |
-C | |
-F | |
-Cl | |
-Br |
The three reactions are determined by the halogen leaving group ability: fluorine is a very poor outgoing group, so it detaches later than hydrogen; chlorine detaches simultaneously with hydrogen and bromine[1, 2].
As the possible products are 2-methyl-2-butene (Zaitsev's rule) and 3-methyl-1-butene (Hofmann's rule), the inductive effect of methyl groups influences the resulting alkene. Only with the E1cB elimination is the second product favored, while in the other two cases it is the first product.
Every reaction involves a different carbon hybridization. In E1cB elimination, as the hydrogen detaches, the carbon is hybridized[3]. In E1 elimination, as the halogen leaves first, another carbon is allowed to take on hybridization. In the E2 elimination, as the detachment of halogen and hydrogen occurs synchronously, both carbons can take on hybridization.
2
sp
2
sp
2
sp
Regardless of the timing of the detachment, when the hydrogen breaks off, it forms a bond with a strong base/weak nucleophile. To simplify the representation, the strong base has been placed outside the plot range and only the bond with the hydrogen can be visualized (colored in dark yellow).
Snapshot 1: after hydrogen detachment, the carbon is hybridized and begins the second reaction phase, in which the halogen breaks off. Due to the destabilizing inductive effect, Hofmann's rule is applied (E1cb)
2
sp
Snapshot 2: hydrogen and halogen detachment happens at the same time and the double bond is formed; Zaitsev's rule is applied (E2)
Snapshot 3: since the second transition state energy is lower, product formation follows Zaitsev's rule (E1)
References
References
[1] H. Hart, L. E. Craine and D. J. Hart, Organic Chemistry: A Short Course, 10th ed., Boston: Houghton Mifflin, Co., 1999.
[2] S. Z. Lavagnino, Eliminazione secondo Hofmann[Video]. (Apr 14, 2022) www.youtube.com/watch?v=nZDIQsNCpjM&list=PLswwssc6Q2yYoP_INHmbmouyxW8oP _Gib&index=47.
[3] J. Ashenhurst. "E1cB–Elimination (Unimolecular) Conjugate Base." Master Organic Chemistry. (Apr 14, 2023) www.masterorganicchemistry.com/2020/02/11/e1cb-elimination-unimolecular-conjugate-base.
External Links
External Links
Permanent Citation
Permanent Citation
D. Meliga, V. Giambrone, L. Lavagnino, S. Z. Lavagnino
"Kinetics of Elimination Reactions"
http://demonstrations.wolfram.com/KineticsOfEliminationReactions/
Wolfram Demonstrations Project
Published: April 21, 2023