Acid-Base Titration with Phenolphthalein Indicator
Acid-Base Titration with Phenolphthalein Indicator
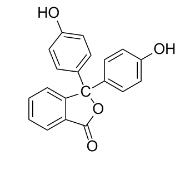
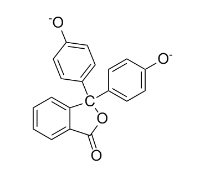
In an acid-base titration, neutralization occurs at pH 7. Since the pH versus concentration curve is so steep around the equivalence point, any indicator that changes color in this general region can be used as an acid-base indicator. Phenolphthalein, one of the most commonly used indicators, shows a transition from colorless to magenta at a pH around 8. As base is added from the buret to the beaker (left), the equilibrium shifts from the protonated molecule (colorless, center) to the deprotonated molecule (magenta, right).
External Links
External Links
Permanent Citation
Permanent Citation
Valerie Keehn, Jill Terpening, Anthony Partacz, Nelson DeLeon
"Acid-Base Titration with Phenolphthalein Indicator" from the Wolfram Demonstrations Project http://demonstrations.wolfram.com/AcidBaseTitrationWithPhenolphthaleinIndicator/
Published: January 15, 2015