Concentration Profile in a Tubular Reactor
Concentration Profile in a Tubular Reactor
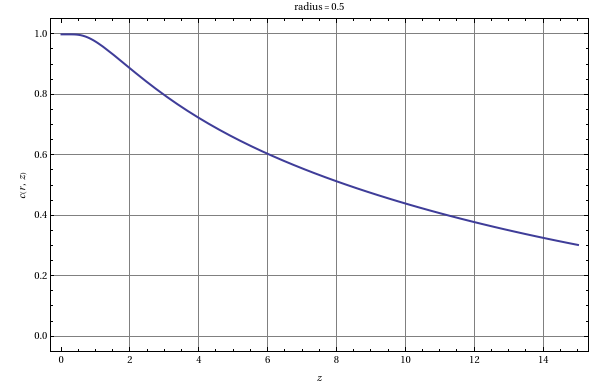
Consider a dilute solution with a solute concentration , in laminar flow. This enters a tubular reactor with a catalytic wall that instantaneously and irreversibly converts the solute to its isomer. The system can be described by the equation [1]:
c
0
v
max
2
r
R
∂c
∂z
1
r
∂
∂r
∂c
∂r
Assume that axial diffusion can be neglected in comparison to axial convection. Here is the maximum laminar parabolic velocity, and are the radial and axial coordinates, respectively, is the reactor radius, is the concentration of the newly formed isomer and is the diffusion coefficient. The boundary conditions are:
v
max
r
z
R
c
c(r,0)=
c
0
c(R,z)=0
∂c(0,z)
∂r
Models like the one described here are useful in obtaining mass transfer data on metallic surfaces in studies designed to prevent or retard the oxidation of iron.