Approximate pH Calculation of Acids
Approximate pH Calculation of Acids
This Demonstration compares approximate and exact values of the for strong and weak acids.
pH
Details
Details
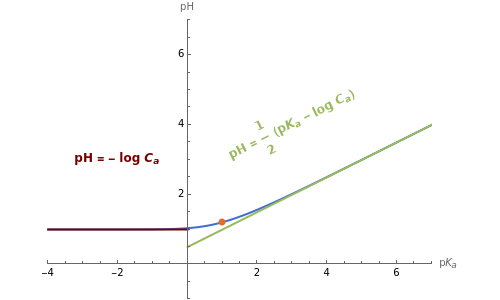
Acids dissociate by the reaction . The initial concentration of acid is and the acid dissociation constant is . This is often expressed as , where logarithms to base 10 are understood. Stronger acids have higher (smaller ) than weaker acids. The acid constant is defined by ==(1-α),with, where is the fraction of protonation. This implies that . Thus given , one can calculate the exact value of ). In this Demonstration, these exact values are shown as blue curves.
HA+
+
H
-
A
HA
C
a
K
a
p=-log
K
a
K
a
K
a
p
K
a
K
a
[][]
+
H
-
A
[HA]
2
(α)
C
a
C
a
[]=[]=α
+
H
-
A
C
a
α
α=
-+
K
a
K
a
4+
C
a
K
a
2
C
a
K
a
pH=-log=-log(α
+
H
C
a
pH
With the strong acid, is very high and , so . This approximation for a strong acid is shown by the red curves, which intersect the axis at .
K
a
α≈1
pH≈-log
C
a
pH
-log
C
a
With the weak acid, is very small compared with , so , thus . This approximation is shown as the green line with slope and intercept at .
K
a
C
a
α≈
C
a
K
a
C
a
pH=(p-log)
1
2
K
a
C
a
1
2
-log
1
2
C
a
We can see that the approximation for a weak acid is not valid at higher concentrations . Thus the approximation for strong acids is only applicable if either or the concentration is small.
C
a
p
K
a
This Demonstration is intended for first year chemistry students (in course LC102, University Pierre Marie Curie, Paris, France).
Permanent Citation
Permanent Citation
Quang-Dao Trinh
"Approximate pH Calculation of Acids"
http://demonstrations.wolfram.com/ApproximatePHCalculationOfAcids/
Wolfram Demonstrations Project
Published: March 7, 2011