
You are using a browser not supported by the Wolfram Cloud
Supported browsers include recent versions of Chrome, Edge, Firefox and Safari.
I understand and wish to continue anyway »
Orbital | Radial |
1s | 0.00519812 -0.0189036rZ 3/2 Z |
2s | 0.000918907 -0.0189036rZ 3/2 Z |
2p | 0.0000100289 -0.0094518rZ 5/2 Z |
3s | 0.0000370511 -0.0063012rZ 3/2 Z 2 r 2 Z |
3p | 0.0000261991 -0.0063012rZ 3/2 Z 2 r 2 Z |
3d | 4.18687× -9 10 -0.0063012rZ 2 r 7/2 Z |
Plot 0.00519812 ^(-0.0189036 r Z) Z^(3/2) from r=0 to 500 for Z=1 | |
Plot[Evaluate[(0.00519812*Z^(3/2))/E^(0.0189036*r*Z)/.{Z->1}],{r,0,500}] | |
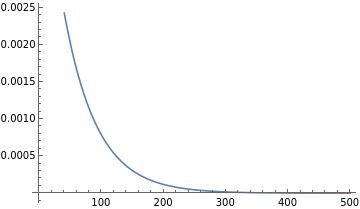
 |
|
 |
|

You are using a browser not supported by the Wolfram Cloud
Supported browsers include recent versions of Chrome, Edge, Firefox and Safari.
I understand and wish to continue anyway »