Jason Sonnenberg, Wolfram Technology Conference, Oct. 31, 2023
A concise guide to harnessing the possibilities of the Wolfram plugin for chemistry.
Cell coloring conventions
Cell coloring conventions
Overall statements.
Take-home message.
Quote from the literature.
(Note that the arXiv ID shows the publication data, e.g. arxiv.org/2307.05782 was published in July.)
(Note that the arXiv ID shows the publication data, e.g. arxiv.org/2307.05782 was published in July.)
ChatGPT textual or code answer.
Screenshot extracted from the quoted literature.
User prompt
...
...
ChatGPT answer URL (ChatGPT September 25 Version)
Chemical Literature
Chemical Literature
The Future of Chemical Education & Research
The Future of Chemical Education & Research
Hopes |
Overcoming Language Barriers |
Personalized Learning |
Real-time Feedback |
Concerns |
Assessment Challenges |
Equitable Accessibility |
Deterministic vs. Probabalistic Tools |
Shortcomings of ChatGPT
Shortcomings of ChatGPT
Issues |
Hopeless at Math |
Makes Stuff Up |
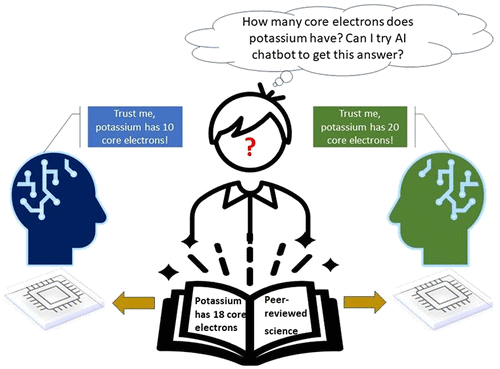
ChatGPT Needs a Chemistry Tutor Too
ChatGPT Needs a Chemistry Tutor Too
Final Exam Results |
Scored 37% or lower on 27 questions |
Independent of question format |
May not be able to explain answer |
Wolfram|Alpha
Wolfram|Alpha
Topical Coverage
Topical Coverage
Acids & Bases, Atoms, Chemical Reactions, Chemical Solutions, Colligative Properties, Concentration, Conversions, Equilibrium, Gases, Kinetics, Molecules, Quantum Chemistry, Structure & Bonding and Thermodynamics
Calculators
Calculators
|
Wolfram Language
Wolfram Language
Chemical Formulas
Chemical Formulas
Chemical Structures
Chemical Structures
Chemical Conversions
Chemical Conversions
Chemical Reactions
Chemical Reactions
BALANCING REACTIONS
BALANCING REACTIONS
SYNTHESIS—BROMINATION OF ALKENES
SYNTHESIS—BROMINATION OF ALKENES
Plugin Usage Examples
Plugin Usage Examples
General Prompts
General Prompts
• It may be helpful to look up the syntax for the chemistry functions that you plan to use before executing code. You don’t need to report what you learn from looking up the syntax.
• When solving chemical equilibrium problems, include an equilibrium table in the output.
• If chemical conversions are required, try Wolfram|Alpha first before writing Wolfram Language code.
• When solving chemical equilibrium problems, include an equilibrium table in the output.
• If chemical conversions are required, try Wolfram|Alpha first before writing Wolfram Language code.
Chemical Structure
Chemical Structure
PHENOL
PHENOL
User prompt
What is the structure of phenol?
What is the structure of phenol?
ALLICIN
ALLICIN
User prompt
Display the space filling, ball and stick, tubes and wireframe plots for allicin next to each other. It may be helpful to look up the syntax for the chemistry functions that you plan to use before executing code. You don’t need to report out what you learn from looking up the syntax.
Display the space filling, ball and stick, tubes and wireframe plots for allicin next to each other. It may be helpful to look up the syntax for the chemistry functions that you plan to use before executing code. You don’t need to report out what you learn from looking up the syntax.
Molecular Properties
Molecular Properties
NORMAL BOILING POINT
NORMAL BOILING POINT
User prompt
Which compound has the lowest normal boiling point? A) HF B) HCl C) HBr D) HI
Which compound has the lowest normal boiling point? A) HF B) HCl C) HBr D) HI
Elemental Composition
Elemental Composition
MULTIPLE CHOICE
MULTIPLE CHOICE
User prompt
Which potassium salt is 28.9% chlorine by mass? a) KCl, b) KClO, C) KClO2, d)KClO3. Be sure to look up any necessary chemical or elemental data and let Wolfram do any math.
Which potassium salt is 28.9% chlorine by mass? a) KCl, b) KClO, C) KClO2, d)KClO3. Be sure to look up any necessary chemical or elemental data and let Wolfram do any math.
HYDROGEN IN ATP
HYDROGEN IN ATP
User prompt
What is the mass percent of hydrogen in ATP?
What is the mass percent of hydrogen in ATP?
Chemical Conversion
Chemical Conversion
SULFUR IN PYRITE
SULFUR IN PYRITE
User prompt
How many sulfur atoms are in 3.00 g of iron pyrite, FeS2? Be sure to look up any necessary chemical or elemental data and let Wolfram do any math.
How many sulfur atoms are in 3.00 g of iron pyrite, FeS2? Be sure to look up any necessary chemical or elemental data and let Wolfram do any math.
MASS TO MOLES WITH UNCERTAINTY
MASS TO MOLES WITH UNCERTAINTY
User prompt
Convert 7.23+-0.0001 g sucrose to moles. Remember that Wolfram Alpha understands uncertainty.
Convert 7.23+-0.0001 g sucrose to moles. Remember that Wolfram Alpha understands uncertainty.
Reaction Balancing
Reaction Balancing
INORGANIC
INORGANIC
User prompt
Balance the chemical reaction Ca(AlO2)2 + HCl -> AlCl3 + CaCl2 + H2O
Balance the chemical reaction Ca(AlO2)2 + HCl -> AlCl3 + CaCl2 + H2O
ORGANIC
ORGANIC
User prompt
Balance the reaction between methane and oxygen to form water and CO2
Balance the reaction between methane and oxygen to form water and CO2
Test Bank Generation
Test Bank Generation
IDEAL GAS LAW
IDEAL GAS LAW
User prompt
I need your help generating multiple versions of homework problems. For each problem, generate three other versions of the question. Keep the same number of significant digits as the original problem and ensure that each digit is different in each problem.
At what temperature does 16.3 g of nitrogen gas have a pressure of 1.25atm in a 25.0 L tank?
What are the answers for the problems? Be sure to look up any necessary chemical or elemental data along with constants and let Wolfram do any math.
I need your help generating multiple versions of homework problems. For each problem, generate three other versions of the question. Keep the same number of significant digits as the original problem and ensure that each digit is different in each problem.
At what temperature does 16.3 g of nitrogen gas have a pressure of 1.25atm in a 25.0 L tank?
What are the answers for the problems? Be sure to look up any necessary chemical or elemental data along with constants and let Wolfram do any math.
Code Assistant
Code Assistant
BUILDING MOLECULE PATTERN OBJECTS
BUILDING MOLECULE PATTERN OBJECTS
User prompt
Read in the usage for MoleculePattern and Bond. Then write code to create an association of five common functional groups where the keys are the group name such as hydroxyl, carbonyl, thiol, etc and the values are the corresponding MoleculePattern for each. When building the molecule pattern objects do not use SMARTS patterns.
Read in the usage for MoleculePattern and Bond. Then write code to create an association of five common functional groups where the keys are the group name such as hydroxyl, carbonyl, thiol, etc and the values are the corresponding MoleculePattern for each. When building the molecule pattern objects do not use SMARTS patterns.
Useful Links
Useful Links