Visualizing Potential Energy Surfaces to
Deepen Chemical Understanding
Visualizing Potential Energy Surfaces to
Deepen Chemical Understanding
Deepen Chemical Understanding
Jason Sonnenberg, PhD (he/him, they/them)
Wolfram|Alpha Chemistry Team
Wolfram|Alpha Chemistry Team
Possible Outside Perception
Possible Outside Perception
Significant Digits
Significant Digits
Compute the mass of 25 mL of water in the 100 mL beaker using the same balance:
In[]:=
Quantity[Around[77.020,0.001],"Grams"]-Quantity[Around[51.034,0.001],"Grams"]
Out[]=
The uncertainty has increased by the expected factor of for two measurements made on a balance with uncertainty δ:
2
In[]:=
Quantity[Around[,δ],"Grams"]-Quantity[Around[,δ],"Grams"]
m
final
m
initial
Out[]=
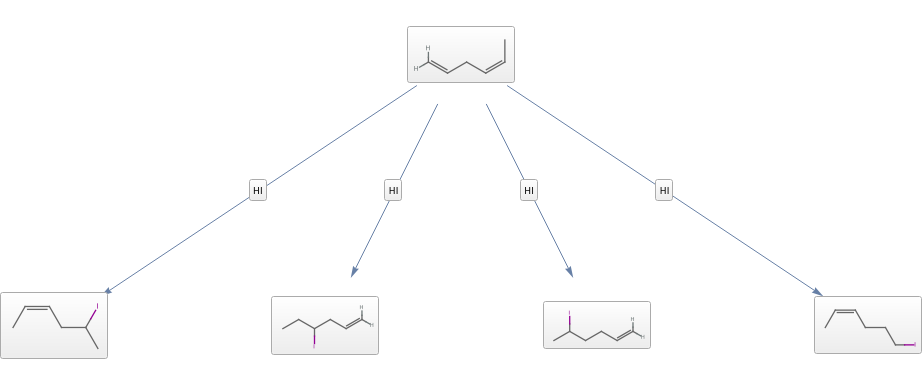
Chemical Conversions
Chemical Conversions
Convert 1.159 grams of aluminum trichloride to millimoles:
In[]:=
ChemicalConvertChemicalInstance,Quantity[Around[1.159,0.001],"Grams"],"Millimoles"
Out[]=
ChemicalInstance,
It’s All About Electrons
It’s All About Electrons
A Molecular Family of Functions
A Molecular Family of Functions
Potential Energy Surfaces: Diatomic Molecules
Potential Energy Surfaces: Diatomic Molecules
Water: One-Dimensional PES
Water: One-Dimensional PES
Water: Two-Dimensional PES
Water: Two-Dimensional PES
Water: Three-Dimensional PES
Water: Three-Dimensional PES
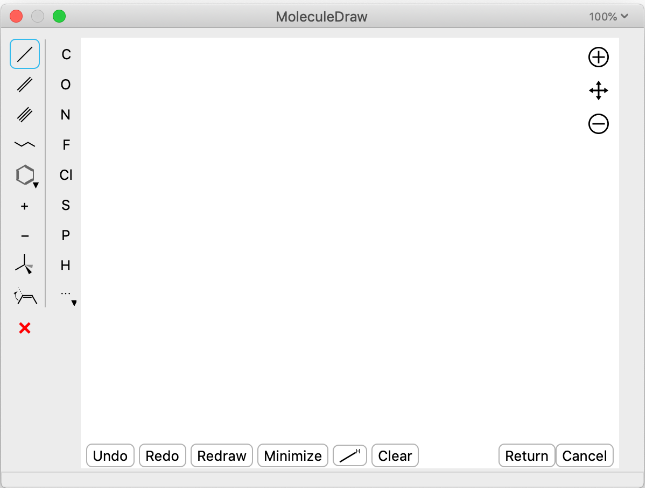
Tools for Chemical Learning Spaces
Tools for Chemical Learning Spaces
Further Information
Further Information
◼
A tutorial is designed to quickly bring all levels of chemistry students up to speed on how to use the Wolfram Language.
◼
A curated portal for chemistry resources in the Wolfram universe.