Heat Capacity of Solids in the Debye Approximation
Heat Capacity of Solids in the Debye Approximation
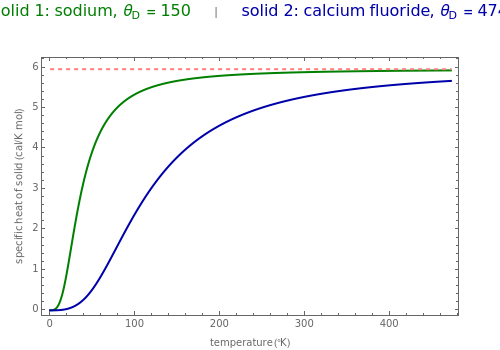
The value of the classical molar heat capacity =3R, depends on temperature. In the Debye approximation, it is given by (T)=9/Tdx, where is the Debye temperature of the solid, is the absolute temperature, and is the gas constant. This Demonstration shows the variation of the specific heat of solids with temperature of representative solids according to the Debye theory.
C
V
C
V
3
R
T
θ
D
θ
D
∫
0
x
e
4
x
2
(-1)
x
e
θ
D
T
R
Details
Details
The dashed red line is the value of the molar heat capacity =3Nk≈6cal/Kmol as given by the Dulong–Petit law. The classical theory for the specific heat of solids does not explain the decrease of specific heat at low temperatures. The physical models of the specific heat curves as given by Einstein and subsequently by Debye employed the quantum theory and agreed well with experiment.
C
V
The Debye model details and the Debye temperature of solids are taken from A. J. Dekker, Solid State Physics, New Delhi: Macmillan India Limited, 2007.
External Links
External Links
Permanent Citation
Permanent Citation
Kallol Das
"Heat Capacity of Solids in the Debye Approximation"
http://demonstrations.wolfram.com/HeatCapacityOfSolidsInTheDebyeApproximation/
Wolfram Demonstrations Project
Published: March 7, 2011