Diffusion and Reaction in a Catalyst Pellet
Diffusion and Reaction in a Catalyst Pellet
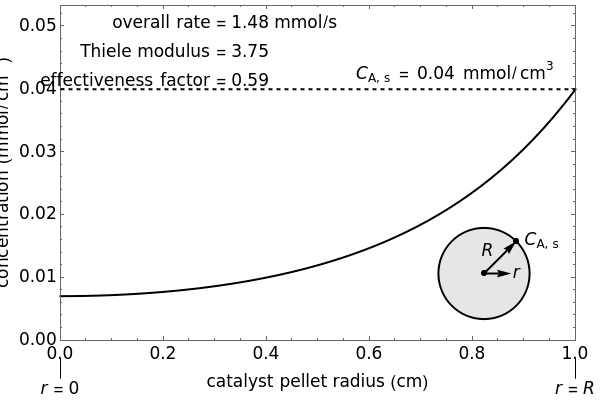
The overall rate of reaction in an isothermal, porous, spherical catalyst pellet is calculated for a first-order, gas-phase reaction that is limited by diffusion in the catalyst pores. This Demonstration plots the reactant concentration inside the catalyst pellet versus the pellet radius. Use the sliders to set the pellet radius, diffusivity and reaction rate constant. The Thiele modulus is a dimensionless number that represents the ratio of reaction rate to diffusion rate. The effectiveness factor is the overall rate of reaction divided by the rate of reaction if the entire catalyst were at =0.04mmol/ (the external surface concentration).
C
A,s
3
cm
Details
Details
For the first-order reaction with rate law =k, the differential equation that describes diffusion and reaction in the catalyst pellet is:
AB
r
A
C
A
2
C
A
2
r
2
r
C
A
r
k
C
A
D
e
where is the radius of the catalyst pellet (); is the concentration in the catalyst (); is the first-order rate constant (); =ϕσ is the effective diffusivity (/s); is the pellet porosity; is the constriction factor; is tortuosity; and , and are set based on typical values for these variables and are unitless.
r
cm
C
A
mmol/
3
cm
k
1/s
D
e
D
AB
τ
2
cm
ϕ
σ
τ
ϕ
σ
τ
The boundary conditions for the differential equation in spherical coordinates are:
C
A
r=0
C
A
C
A,s
r=R
where is the pellet radius () and is the concentration () at .
R
cm
C
A,s
mmol/
3
cm
r=R
The differential equation in dimensionless form is:
2
2
λ
2
λ
Ψ
λ
2
Φ
Φ=R
k/
D
e
where and . The Thiele modulus is the dimensionless ratio of the surface reaction rate to the diffusion rate.
Ψ=
C
A
C
A,s
λ=r/R
Φ
The boundary conditions in dimensionless form are:
Ψ=finite
λ=0
Ψ=1
λ=1
The solution to the differential equation in dimensionless form is:
Ψ=cosh(Φλ)+sinh(Φλ)
c
1
λ
c
2
λ
where and are constants that are obtained using the boundary conditions. The solution becomes:
c
1
c
2
Ψ=
1
λ
sinh(Φλ)
sinhΦ
and substituting the dimensionless variables into the solution yields:
C
A
C
A,s
R
r
sinh(Φr/R)
sinhΦ
The overall rate of reaction is:
M
A
M
A
D
e
C
A,s
Ψ
λ
λ=1
where =ΦcothΦ-1,
Ψ
λ
λ=1
substituting this into the solution yields:
M
A
D
e
C
A,s
where has units of .
M
A
mmol
s
The effectiveness factor is the reaction rate in the pellet divided by the reaction rate at the surface:
η
η=
M
A
M
A,s
where =-π=kπ.
M
A,s
r
A,s
4
3
3
R
C
A,s
4
3
3
R
Thus, is:
η
η=(ΦcothΦ-1)
4πR
D
e
C
A,s
kπ
C
A,s
4
3
3
R
which reduces to:
η=
3
Φ(ΦcothΦ-1)
References
References
[1] H. S. Fogler, Elements of Chemical Reaction Engineering, 4th ed., Upper Saddle River, NJ: Prentice Hall, 2006 pp. 813–852.
Permanent Citation
Permanent Citation
Rachael L. Baumann, John L. Falconer
"Diffusion and Reaction in a Catalyst Pellet"
http://demonstrations.wolfram.com/DiffusionAndReactionInACatalystPellet/
Wolfram Demonstrations Project
Published: January 1, 1999