Endpoints Method for Predicting Chemical Degradation in Frozen Foods
Endpoints Method for Predicting Chemical Degradation in Frozen Foods
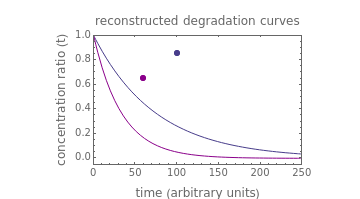
The chemical degradation of nutrients and pigments in stored frozen foods frequently follows first-order or approximately first-order kinetics. If their concentration ratios after storage for times and at temperatures and , respectively, are experimentally determined, then it is possible, at least in theory, to extract their kinetic parameters from these endpoints, assuming that the rate constant’s temperature dependence follows the simple exponential model (a convenient substitute for the traditional Arrhenius equation).
t
1
t
2
T
1
T
2
This Demonstration exploits this principle to recreate the entire degradation curves at the two temperatures (both must be below the freezing point) and predict those at other frozen temperatures by numerical solution of the two corresponding simultaneous nonlinear equations. The solution is aided by matching the two endpoints with their recreated degradation curves on the screen, using sliders.