Daniell Cell: Charging and Discharging
Daniell Cell: Charging and Discharging
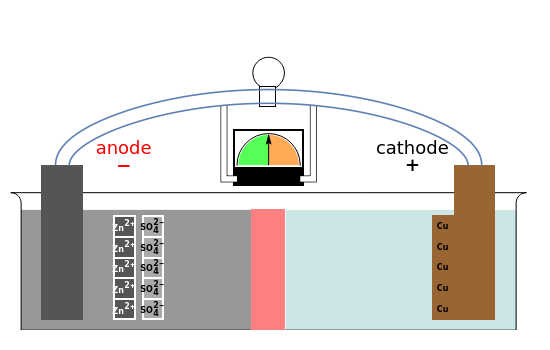
This Demonstration shows the motions of the , and ions in a Daniell cell: . The cell can function either as a battery or as an electrolytic cell. Two half-cells, one made of copper filled with copper sulfate and the other made of zinc filled with zinc sulfate, are connected through a porous glass partition. Electroneutrality is maintained by the moving sulfate ions. The initial condition is a homogeneous distribution of all the ions with no polarization of the electrodes.
2+
Cu
2+
Zn
2-
SO
4
Zn|(1M)||(1M)|Cu
2+
Zn
2+
Cu
You can use the checkbox to show all the species in solution or just the ones responsible for current flow. As in the real thing, every step takes place almost simultaneously, so time is divided into steps for better understanding of the process.
After preparing the solution and closing the circuit, the following half reactions take place in the Daniell cell:
Zn(s)(aq)+2
2+
Zn
-
e
Cu
2
-
e
The ions must move through the partition to preserve neutrality[1].
2-
SO
4
By attaching a compatible voltage source to the electrolyte cell, the reverse process occurs. During both charging and discharging, the concentrations vary, as indicated by color changes in the solution.
Details
Details
Snapshot 1: after mixing the salts in each solution and closing the circuit, electromotive force (emf) is generated
Snapshot 2: after applying electric current, concentrations in the solutions change: the zinc solution becomes less concentrated while the copper solution becomes more concentrated
Snapshot 3: after applying an emf, ions are reduced and is deposited on the plate, while ions are formed as oxidation takes place
2+
Zn
Zn
2+
Cu
References
References
[1] A. da Rosa, "Daniell Cell," Fundamentals of Renewable Energy Processes, 3rd ed., Amsterdam: Elsevier/AP, 2013. (Jun 16, 2021) www.sciencedirect.com/topics/engineering/daniell-cell.
External Links
External Links
Permanent Citation
Permanent Citation
A. Ratti, D. Meliga, L. Lavagnino, S. Z. Lavagnino
"Daniell Cell: Charging and Discharging"
http://demonstrations.wolfram.com/DaniellCellChargingAndDischarging/
Wolfram Demonstrations Project
Published: June 30, 2021