An Alternative Periodic Table
An Alternative Periodic Table
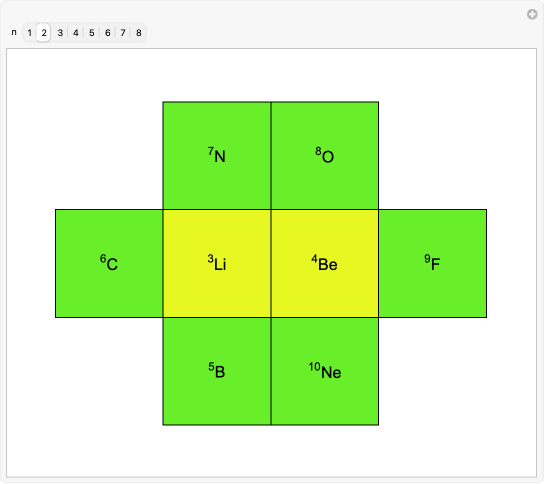
This version of the periodic table shows periodicity using quantum numbers. Each plate is arranged according to the principal quantum number . Different colors represent different subshells , , , , that is, values of quantum number. Yellow stands for -orbital for alkali metals and alkaline earths; green for -orbitals, that is, the case of nonmetals and semiconductors; pink for -orbitals corresponding to transition metals; and blue for -orbitals in lanthanides and actinides. Moving from top to bottom represents the quantum number and right to left the quantum number .
n
s
p
d
f
l
s
p
d
f
m
s
Details
Details
For more information, see the Timmothy Stowe periodic table in the Wikipedia entry for "Alternative periodic tables".
External Links
External Links
Permanent Citation
Permanent Citation
Enrique Zeleny
"An Alternative Periodic Table"
http://demonstrations.wolfram.com/AnAlternativePeriodicTable/
Wolfram Demonstrations Project
Published: October 12, 2011