Attainable Regions for Maleic Anhydride Manufacture in a Plug-Flow Reactor
Attainable Regions for Maleic Anhydride Manufacture in a Plug-Flow Reactor
The synthesis of maleic anhydride, , involves the following reactions in the presence of a vanadium pentoxide catalyst:
C
4
H
2
O
3
C
6
H
6
9
2
O
2
C
4
H
2
O
3
CO
2
H
2
C
4
H
2
O
3
O
2
CO
2
H
2
C
6
H
6
15
2
O
2
CO
2
H
2
If air is supplied in excess, the concentration of oxygen can be considered as constant and the reaction rates are given by =, =, and =, where is the rate constant of reaction (expressed in kg catalyst), is the concentration of benzene, and is the concentration of maleic anhydride.
r
1
k
1
C
A
r
2
k
2
C
P
r
3
k
3
C
A
k
i
i
3
m
-1
s
C
A
C
P
The temperature-dependent rate constants are given by =4280, =70100, and =26.
k
1
-12660
T
e
k
2
-15000
T
e
k
3
-10800
T
e
The governing equations at steady-state, obtained by writing molar balances, for a packed-bed reactor modelled as a PFR (plug-flow reactor) are
v
0
d
C
A
dW
k
1
C
A
k
3
C
A
C
A
3
m
v
0
d
C
P
dW
k
1
C
A
k
2
C
P
C
P
v
0
3
m
W
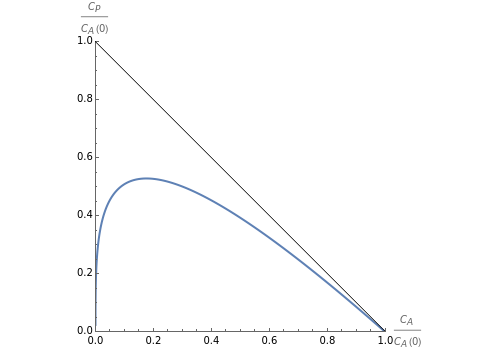
The final/outlet concentrations of benzene ) and maleic anhydride ) are obtained by solving the steady-state equations numerically, with the weight of the catalyst ranging from 0 to 10,000 kg.
(
C
A
(
C
P
This Demonstration plots the attainable region for maleic anhydride synthesis for values of the temperature fixed by the user.