Formulas and Structures for Some Simple Molecules
Formulas and Structures for Some Simple Molecules
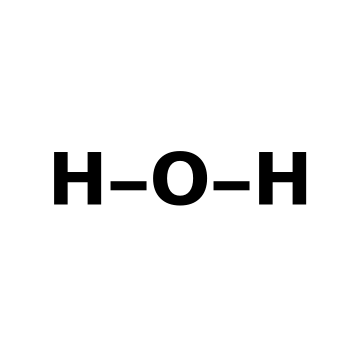
A chemical formula specifies the number of atoms of each element in a compound. The connection formula shows the topology, how the atoms are connected by chemical bonds, usually without regard to their geometry. The actual geometric shape of a molecule can be represented by a structure diagram. This also includes the location of unshared pairs of electrons, which strongly influence the geometry. A Natta projection shows a bond projecting toward the viewer as a solid wedge, while a receding bond is shown as a dashed wedge. In this Demonstration, you can also chose to view a "ball and stick" molecular model. Beginning students in chemistry should become familiar with all these alternative descriptions of molecular structure.
Details
Details
Snapshot 1: connection diagram for ammonia showing a nitrogen atom connected to three hydrogen atoms
Snapshot 2: repulsion by the unshared pair of electrons causes the three N-H bonds to be arranged in a trigonal pyramid
Snapshot 3: ball and stick model of ammonia molecule
Permanent Citation
Permanent Citation
S. M. Blinder
"Formulas and Structures for Some Simple Molecules"
http://demonstrations.wolfram.com/FormulasAndStructuresForSomeSimpleMolecules/
Wolfram Demonstrations Project
Published: March 7, 2011