Simple Reaction with Segregation in a Batch Reactor
Simple Reaction with Segregation in a Batch Reactor
Consider a simple chemical reaction in a batch reactor. The reaction rate in terms of the intensity of mixing, , is given by: =-=-k-I, where and are the instantaneous concentrations, and are the mean concentrations, and and are the initial concentrations of species and . The governing equation is the following:
A+B→C
I
-
r
A
c
A
c
B
-
c
A
-
c
B
-
c
A0
-
c
B0
c
A
c
B
-
c
A
-
c
B
-
c
A0
-
c
B0
A
B
d()
-
c
A
dt
-
r
A
This equation can be written in dimensionless form as:
dy
dθ
D
a
η=
-
c
B0
-
c
A0
θ=
t
τ
m
τ
m
y=
-
c
A
-
c
A0
D
a
-
c
A0
τ
m
I=
-
t
τ
m
e
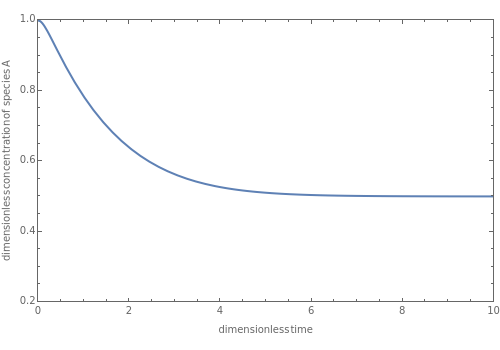
This Demonstration displays the dimensionless concentration versus the dimensionless time for various values of the Damköhler number and the initial concentration ratio. It is straightforward to see that the steady-state dimensionless concentration is independent of the Damköhler number. The Damköhler number has an influence only on how fast this steady-state dimensionless concentration is reached. This steady-state dimensionless concentration is equal to =1-η. When →∞, there is an analytical expression for the dimensionless concentration, which is given by:
y
ss
D
a
y
∞
-(η-1)++4ηI
2
(η-1)
2
I=
-θ
e