Magic Numbers in the Nuclear Shell Model
Magic Numbers in the Nuclear Shell Model
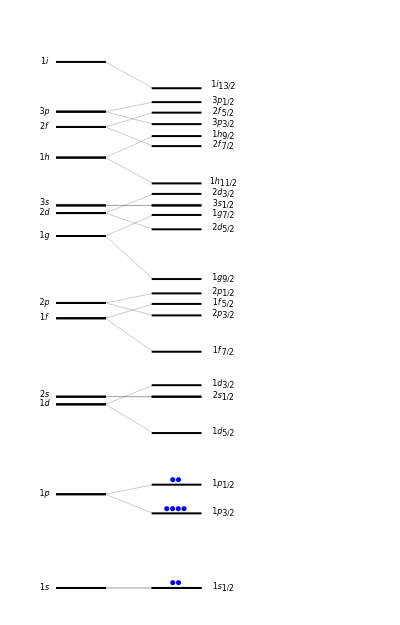
The nuclear shell model is an analog of the Aufbau principle, which describes the electronic structure of atoms. It was developed independently in 1949 by Maria Goeppert-Mayer and by J. Hans D. Jensen and coworkers. Goeppert-Mayer and Jensen shared the 1963 Nobel Prize in physics. According to the shell model, nuclear energy levels, individually for neutrons and protons, are filled successively, in conformity with the Pauli exclusion principle. Just as in the atomic case, there are certain "magic numbers" in the occupancy of nucleon shells: 2, 8, 20, 28, 50, 82, and 126, which confer enhanced stability to nuclei. (This is analogous to the atomic magic numbers 2, 10, 18, 36, 54, 86.)
To a first approximation, the energy levels of nucleons begin with those of an isotropic three-dimensional harmonic oscillator. A second approximation lowers each level slightly by an amount proportional to , where is the orbital angular momentum quantum number. The code used to designate the values is s, p, d, f, g, h, i, k, … (sober physicists don't find giraffes hiding in kitchens). In contrast to the convention used in atoms, the integer quantum number labels the ordering of levels of a given angular momentum. Thus you will find levels labeled 1, 2, etc. The left side of the graphic gives the designations of the levels. It was suggested by Enrico Fermi that spin-orbit coupling plays an important role in determining nucleon energies. Each -level, except , is accordingly split into two states, with . This leads finally to the spectrum of nucleon energy levels shown on the right, with appropriate gaps determining the magic numbers. Neutrons are represented by blue dots and protons by red dots. The diagram is highly schematic since the magnitudes and even the ordering of the levels differ from nucleus to nucleus.
2
l
l
l=0,1,2,3,…
p
d
nl
l
l=0
j=l±
1
2
n
l
j
Following is a partial listing of nuclides with magic numbers and/or :
N
Z
N=2
4
2
N=8
15
7
16
8
N=20
36
16
37
17
38
18
39
19
40
20
N=28
48
20
50
22
51
23
52
24
54
26
N=50
86
36
88
38
89
39
92
42
N=82
136
54
138
56
139
57
140
58
141
59
142
60
144
62
N=126
208
82
Z=2
3
2
4
2
Z=8
A
8
A=16,17,18
Z=20
A
20
A=40,42,43,44,46,48
Z=28
A
28
A=58,60,61,62,64
Z=50
A
50
A=112,114,115,116,118,120,122,124
Z=82
A
82
A=204,206,208.
Of special interest are the doubly-magic nuclei: He, O, Ca, Ca, and Pb.
4
2
16
8
40
20
48
20
208
82