Pauling Electronegativity and Dipole Moment
Pauling Electronegativity and Dipole Moment
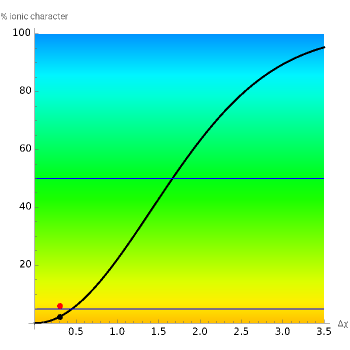
This Demonstration shows the relationship between the dipole moment of molecules and Pauling's electronegativity. Pauling defined electronegativity[1] and proposed the following relation: . This is an empirical approximation and sometimes is not accurate. More recent approaches are more complex and depend on other electronic properties of the molecule[2].
%ioniccharacter=1-
1
4
2
(-)
χ
a
χ
b
e
The "experimental" window uses some experimental data and compares them with the theoretical prediction. An ionic bond does not mean necessarily 100% ionic character; anything above 50% is sufficient to classify the bond as ionic. When the bond is ionic, according to the experimental value, one element is colored red (positive charge) and the other one blue (negative charge). In the case of a polar covalent bond, a segment links both elements to indicate the electron pair shared between the two atoms. The color intensity is lighter than the ionic bond as the electronic distribution is asymmetrical[3]. Moving the "dipole" slider gives a new example with the predicted value shown.
The "theoretical" window shows the plot associated with the bonding molecular orbital distribution of two generic orbitals. Three areas are highlighted according only to the electronegativity difference: nonpolar, polar covalent and ionic bond[4].
s
Details
Details
Snapshot 1: Discrepancy between experimental and theoretical percentage of ionic character of the CsF molecule. The predicted value is lower than the experimental one, but this is still classified as an ionic bond.
Snapshot 2: Theoretical model of a polar covalent bond. Bonding electrons are shared unequally between the atoms forming partial charges () on the atoms.
δ
Snapshot 3: Theoretical model of a ionic bond. Ions are formed as a consequence of the complete migration of one electron. The charge displacement is neat in this case.
References
References
[1] LibreTexts. "Electronegativity and Dipole Moment." (Jan 14, 2020) chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Map%3A_Physical_Chemistry_for_the_Biosciences_(Chang)/12%3A_The_Chemical_Bond/12.4%3A_Electronegativity_and_Dipole_Moment.
[2] Quora. "What Is the Relation of Ionic Character with Anionic Charge?" (Jan 14, 2020) www.quora.com/What-is-the-relation-of-ionic-character-with-anionic-charge.
[3] "9.3 Polarity of Molecules." (Jan 14, 2020) wps.prenhall.com/wps/media/objects/3081/3155729/blb0903.html.
[4] StackExchange. "Why Are Bonds Ionic when the Electronegativity Difference between Bonded Atoms Is Greater than 1.7?" (Jan 14, 2020) chemistry.stackexchange.com/questions/9222/why-are-bonds-ionic-when-the-electronegativity-difference-between-bonded-atoms-i.
External Links
External Links
Permanent Citation
Permanent Citation
D. Meliga, S. Z. Lavagnino
"Pauling Electronegativity and Dipole Moment"
http://demonstrations.wolfram.com/PaulingElectronegativityAndDipoleMoment/
Wolfram Demonstrations Project
Published: January 20, 2020