Electronegativity and Bonding Type
Electronegativity and Bonding Type
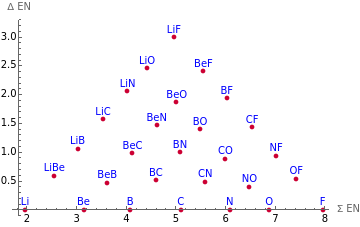
The first column of the table contains symbols for compounds of elements within a chosen period (whether or not they exist or what formulas they have). The third and fourth columns of the table give the sum and difference of the electronegativity for the elements in the compounds. The plotted pairs of the compounds form a triangle. The types of bonding (metallic bond/covalent bond/ionic bond) are localized around the three corners of the triangle, with the transitional types between them.
(ΣEN,ΔEN)
Details
Details
Metals have low values of electronegativity and therefore sums and differences of these values are small. They can be found around the left corner of the triangle (metallic bonding).
Non-metals have relatively high values of electronegativity and therefore high sums and low differences of these values. They can be found around the right corner of the triangle (covalent bonding).
Salts are compounds of metals and non-metals. The differences of the electronegativity values are high. They can be found around the top of the triangle (ionic bonding).
References
References
H. Wambach (ed.), "Atome-Bindungen-Strukturen," Materialien-Handbuch Kursunterricht Chemie, Band 1, 1996.
External Links
External Links
Permanent Citation
Permanent Citation
Guenther Gsaller, Heinz Wambach
"Electronegativity and Bonding Type"
http://demonstrations.wolfram.com/ElectronegativityAndBondingType/
Wolfram Demonstrations Project
Published: March 7, 2011