Temperature Dependence of Henry's Law Constant
Temperature Dependence of Henry's Law Constant
Henry's law was originally formulated by William Henry in 1803. This law describes the solubility of gases in liquids. Henry's law states that at constant temperature, the mole fraction of a given gas dissolved in the liquid is directly proportional to the partial pressure of that gas (i.e., , where is the total pressure, is the Henry's law constant, and is the vapor-phase mole fraction).
x
Py=Hx
P
H
y
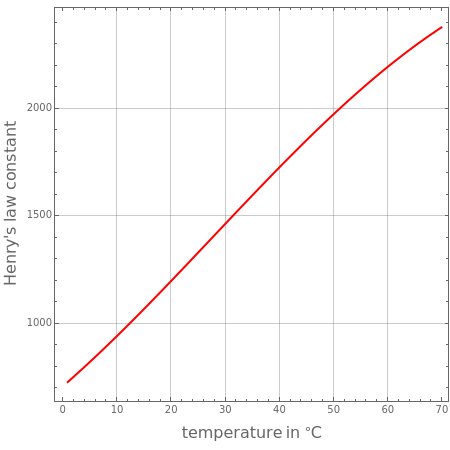
The constant in Henry's law changes with the system's temperature . This is why it is preferable to name it Henry's coefficient. Multiple equations take into account the effect of temperature on this constant. Here, the following relationship is adopted[1]:
T
H=1/exp(A+B/T+Cln(T)+DT)
T
A
B
C
D
The present Demonstration plots for different gaseous species in water ( is expressed in atm).
H(T)
H
References
References
[1] R. H. Perry, Perry’s Chemical Engineers’ Handbook, 4th ed., New York: McGraw–Hill, 1963.
Permanent Citation
Permanent Citation
Housam Binous, Ahmed Bellagi
"Temperature Dependence of Henry's Law Constant"
http://demonstrations.wolfram.com/TemperatureDependenceOfHenrysLawConstant/
Wolfram Demonstrations Project
Published: June 9, 2015