Volatility Inversion in Extractive Distillation
Volatility Inversion in Extractive Distillation
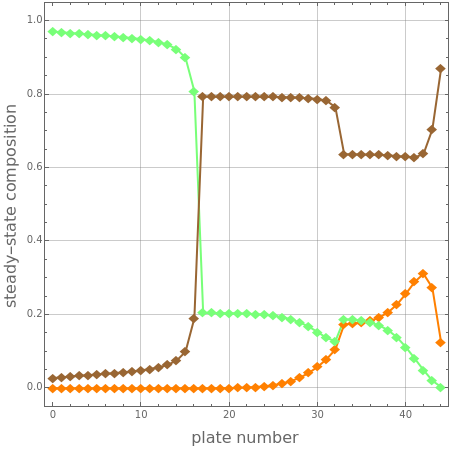
Consider an extractive distillation column operating at atmospheric pressure with 43 stages, a partial reboiler, and a total condenser. It is used to separate acetone and methanol using chlorobenzene as an entrainer.
Pure entrainer at is fed to the column at a flow rate, , to be set by the user at stage 17 (counting from the top). The lower feed is composed of an equimolar mixture of acetone and methanol. The lower feed, at , is located at stage 33 (counting from the top) and has a flow rate equal to .
320K
FU
320K
540kmole/hr
The Demonstration solves the MESH equations (mass,equilibrium, summation, and heat) and displays the composition and temperature profiles for user-set values of the reflux ratio, . The second degree of freedom is set by taking a distillate flow rate equal to .
RR
270kmole/hr
For this particular entrainer selection, one observes a phenomenon called volatility inversion. Indeed, methanol (b.p. ) exits the extractive distillation column as a pure distillate product, while the more volatile component acetone (b.p. ) leaves accompanied by the entrainer (i.e., chlorobenzene) at the bottom of the column.
64.65°C
56.05°C
Details
Details
The mixture is assumed to obey modified Raoult's law, and activity coefficients are predicted using the Wilson model.
References
References
[1] M. F. Doherty and M. F. Malone, Conceptual Design of Distillation Systems, Boston: McGraw-Hill, 2001.
[2] E. J. Henley and J. D. Seader, Equilibrium-Stage Separation Operations in Chemical Engineering, New York: Wiley, 1981.
[3] W. L. Luyben and I.-L. Chien, Design and Control of Distillation Systems for Separating Azeotropes, Hoboken, NJ: Wiley, 2010.
Permanent Citation
Permanent Citation
Housam Binous, Naim Faqir
"Volatility Inversion in Extractive Distillation"
http://demonstrations.wolfram.com/VolatilityInversionInExtractiveDistillation/
Wolfram Demonstrations Project
Published: July 18, 2012