Residue Curve Map for Methyl Acetate and Isopropyl Acetate Chemistry at 1 atm
Residue Curve Map for Methyl Acetate and Isopropyl Acetate Chemistry at 1 atm
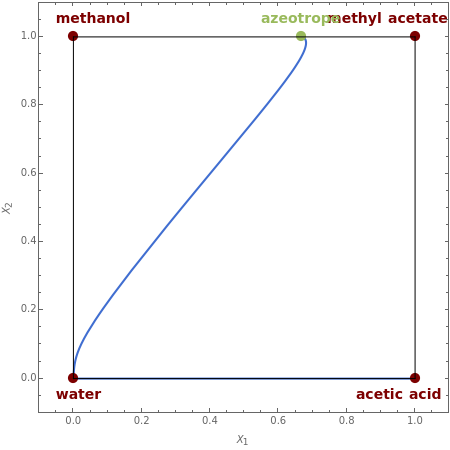
This Demonstration plots the residue curve passing through the locator position.
The first system considered is the esterification of acetic acid with methanol to produce methyl acetate and water:
CCOOH+COH⇌CCOOC+O
H
3
H
3
H
3
H
3
H
2
There is a nonreactive azeotrope between methanol and methyl acetate corresponding to =0.669 and =1. This azeotrope is an unstable node. Acetic acid is the stable node. Water, methyl acetate, and methanol are three saddle points.
X
1
X
2
The axes labels are and , the independent liquid transformed compositions given by=+ and =+, where is the liquid mole fraction of component and methyl acetate is the reference component.
X
1
X
2
X
1
x
aceticacid
x
methylacetate
X
2
x
methanol
x
methylacetate
x
A
A
The second system considered is the esterification of acetic acid with isopropanol to produce isopropyl acetate and water:
CCOOH+CCH(OH)C⇌CCOOC+O
H
3
H
3
H
3
H
3
H(C)
H
3
2
H
2
If you select this second option, the Demonstration again plots the residue curve passing through the locator position.
There is a reactive azeotrope corresponding to =0.231 and =0.748. This reactive azeotrope is an unstable node. In addition, there are two nonreactive binary azeotropes. The first is between isopropanol and water, while the other is between isopropanol and isopropyl acetate. Both nonreactive azeotropes are saddle points. Isopropyl acetate and water are two saddle points while acetic acid and isopropanol are two stable nodes.
X
1
X
2
The axes labels are and , with the independent liquid transformed compositions given by =+ and =+, where is the liquid mole fraction of component and isopropyl acetate is the reference component.
X
1
X
2
X
1
x
aceticacid
x
isopropylacetate
X
2
x
isopropanol
x
isopropylacetate
x
A
A
Details
Details
For decades, reactors were followed by separation units such as distillation columns in order to recycle reactants and to separate products. Reactive distillation, which combines both operations in one piece of equipment, presents many advantages: increased selectivity, lower capital investment and operating cost, higher conversion in the case of equilibrium-limited reactions (such as esterification reactions considered in the present Demonstration), decreased risks of thermal runaway, separation of close-boiling compounds and reduction of environmental emissions, and so on.
The computation and analysis of residue curve maps is an important tool in the conceptual design of reactive distillation columns.
The governing equation is the following differential-algebraic system:
d
X
i
dτ
X
i
Y
i
τ
i=1,…,-1
N
c
i≠k
k
N
c
X
i
Y
i
X
i
x
i
ν
i
ν
k
x
k
1-
ν
T
ν
k
x
k
Y
i
y
i
ν
i
ν
k
y
k
1-
ν
T
ν
k
y
k
i=1,…,-1
N
c
i≠k
x
i
y
i
i
k
ν
i
i
ν
T
i=
N
c
∑
i=1
ν
i
The computation takes into account the deviation from ideal behavior in the liquid phase by using the Wilson model for the methyl acetate chemistry and the NRTL model for the isopropyl acetate chemistry as well as acetic acid dimerization in the gas phase by using Marek's method (1954 and 1955).
For more information, see
M. F. Doherty, M. F. Malone, Conceptual Design of Distillation Systems, New York: McGraw-Hill, 2001.
H. Binous, "Residue Curve Map for Homogeneous Reactive Quaternary Mixtures," Computer Applications in Engineering Education, 15(1), 2007 pp. 73–77.
Permanent Citation
Permanent Citation